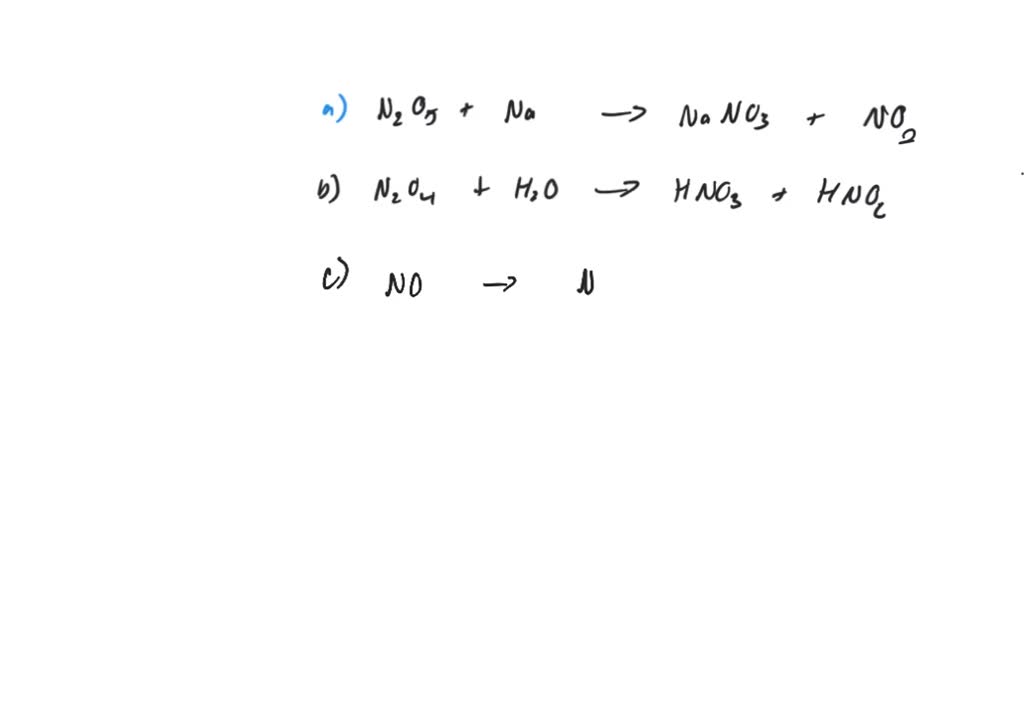Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas . Nitrogen is heated with oxygen to form dinitrogen. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Write a balanced equation for each of the following combination reactions. Express your answer as a chemical equation. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Given the following diagram of available. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only 2 n atoms on the reactant side. N 2 o is a strong oxidizing agent that decomposes. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide.
from www.numerade.com
Given the following diagram of available. Nitrogen is heated with oxygen to form dinitrogen. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only 2 n atoms on the reactant side. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. N 2 o is a strong oxidizing agent that decomposes. Express your answer as a chemical equation. Write a balanced equation for each of the following combination reactions.
SOLVED7. BALANCE EQUATIONS (10 MARKS) a. Reaction of nitrogen gas with
Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Given the following diagram of available. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Nitrogen is heated with oxygen to form dinitrogen. Write a balanced equation for each of the following combination reactions. N 2 o is a strong oxidizing agent that decomposes. After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only 2 n atoms on the reactant side. Express your answer as a chemical equation. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3).
From www.numerade.com
SOLVED Part A Nitrogen is heated with oxygen to form dinitrogen Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Express your answer as a chemical equation. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Write a balanced equation for each of the following combination reactions. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.alamy.com
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen dioxide Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Write a balanced equation for each of the following combination reactions. After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only 2 n atoms on the reactant side. Nitrogen is heated with oxygen to form dinitrogen. Given the following diagram of available. Express. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED7. BALANCE EQUATIONS (10 MARKS) a. Reaction of nitrogen gas with Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Express your answer as a chemical equation. Write a balanced equation for each of the following combination reactions. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Nitrogen is heated with oxygen to form dinitrogen. N 2 o. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.chegg.com
Solved nitrogen dioxide gas reacts with nitrogen trioxide Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Express your answer as a chemical equation. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Nitrogen is heated with oxygen to form dinitrogen. After balancing the oxygen. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED Dinitrogen pentoxide gas to form nitrogen dioxide Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). N 2 o is a strong oxidizing agent that decomposes. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Nitrogen is heated with oxygen to form. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.chegg.com
Solved Consider the reaction between nitrogen and oxygen gas Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Given the following diagram of available. Write a balanced equation for each of the following combination reactions. Express your answer as a chemical equation. After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only 2 n atoms on the reactant side. N 2 o is a strong oxidizing agent that decomposes. Nitrogen is. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED Dinitrogen pentoxide in the gas phase to form Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrogen is heated with oxygen to form dinitrogen. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Given the following diagram of available. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. N 2 o is a strong oxidizing agent that decomposes. Write a balanced equation for. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
Dinitrogen trioxide, a blue solid, dissociates to form nitrogen Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Given the following diagram of available. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Determine the correct chemical formulas for the reactants and. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED n particular reaction 90g of dinitrogen trioxide gas (NzOs) was Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.chegg.com
Solved Gaseous Nitrogen Dioxide NO2, Reacts With Oxygen T... Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Nitrogen is heated with oxygen to form dinitrogen trioxide gas. N 2 o is a strong oxidizing agent that decomposes. Given the following diagram of available.. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.chegg.com
Solved Nitrogen monoxide gas reacts with oxygen gas to form Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrogen is heated with oxygen to form dinitrogen trioxide gas. After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only 2 n atoms on the reactant side. Express your answer as a chemical equation. Nitrogen is heated with oxygen to form dinitrogen. Write a balanced equation for the reaction of molecular nitrogen (n. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED The gases nitrogen dioxide and oxygen react to produce Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Express your answer as a chemical equation.. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.youtube.com
How to Balance N2 + O2 = NO2 (Nitrogen gas + Oxygen gas) YouTube Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Express your answer as a chemical equation. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. Determine the correct chemical formulas for the reactants and the product. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.studyxapp.com
dinitrogen tetraoxide and nitrogen dioxide are two gases that exist in Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Express your answer as a chemical equation. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. N 2 o is a strong oxidizing agent that decomposes. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Given the following diagram of available. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Determine the. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED Pure nitrogen dioxide (NO2) forms when dinitrogen oxide (N2O Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Write a balanced equation for each of the following combination reactions. Express your answer as a chemical equation. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED Dinitrogen pentoxide gas is produced by the reaction of Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Given the following diagram of available. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen pentoxide. Nitrogen is heated with oxygen to form dinitrogen. Nitrous oxide resembles oxygen in. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From www.numerade.com
SOLVED Nitrogen and oxygen gases react to form dinitrogen trioxide gas Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas Nitrogen is heated with oxygen to form dinitrogen. Nitrogen (n2) gas reacts with oxygen (o2) gas to produce dinitrogen trioxide (n2o3). Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. N 2 o is a strong oxidizing agent that decomposes. After balancing the oxygen atoms, the product side now has 4 nitrogen atoms and there are only. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas N 2 o is a strong oxidizing agent that decomposes. Express your answer as a chemical equation. Nitrogen is heated with oxygen to form dinitrogen trioxide gas. Nitrogen is heated with oxygen to form dinitrogen. Determine the correct chemical formulas for the reactants and the product for the reaction between nitrogen and oxygen forming dinitrogen trioxide. Given the following diagram. Nitrogen Is Heated With Oxygen To Form Dinitrogen Trioxide Gas.